Material Selection and Preparation for Medical Device Housings
Choosing the right material is crucial for CNC machining medical device housings. The selected material must meet specific criteria to ensure the final product's functionality, durability, and compliance with medical standards.
Biocompatible Materials for Medical Applications
When selecting materials for medical device housings, biocompatibility is paramount. Materials must not cause adverse reactions when in contact with human tissue or bodily fluids. Common biocompatible materials include:
- Medical-grade stainless steel (e.g., 316L)
- Titanium and its alloys
- High-performance plastics like PEEK (Polyetheretherketone)
- Medical-grade aluminum alloys
These materials offer excellent strength-to-weight ratios, corrosion resistance, and compatibility with sterilization processes.
Material Properties and Performance Considerations
Beyond biocompatibility, consider the following properties when selecting materials for CNC machining medical device housings:
- Mechanical strength and durability
- Thermal stability
- Chemical resistance
- Electrical insulation (if required)
- Ability to withstand sterilization processes
Evaluate these properties in the context of the device's intended use and operating environment to ensure optimal performance.
Material Preparation and Handling
Proper material preparation is essential for achieving high-quality CNC machined medical device housings. Follow these best practices:
- Store materials in a clean, controlled environment to prevent contamination
- Inspect materials for defects or irregularities before machining
- Use appropriate cleaning and degreasing techniques to prepare surfaces
- Handle materials with clean gloves to prevent oils and contaminants from transferring
By carefully selecting and preparing materials, manufacturers can ensure the foundation for producing high-quality medical device housings through CNC machining.

Design Considerations for CNC Machined Medical Device Housings
Effective design is critical for creating medical device housings that meet functional requirements, regulatory standards, and manufacturability criteria. Consider the following aspects when designing for CNC machining:
Designing for Functionality and Ergonomics
Medical device housings must be designed with both function and user experience in mind. Key considerations include:
- Proper sizing and dimensions to accommodate internal components
- Ergonomic shapes for comfortable handling by medical professionals
- Adequate ventilation to prevent overheating of electronic components
- Incorporation of mounting points for internal hardware
- Design of cable management systems and access ports
Collaborate closely with medical professionals and end-users to ensure the design meets practical needs in clinical settings.
Optimizing for CNC Machining Processes
To maximize efficiency and quality in CNC machining medical device housings, consider these design optimization strategies:
- Use standard tool sizes and avoid deep pockets to reduce machining time
- Design with uniform wall thicknesses to prevent warping
- Incorporate fillets and rounds to reduce stress concentrations
- Avoid sharp internal corners that are difficult to machine
- Consider the need for fixturing and workholding during machining
By optimizing designs for CNC machining, manufacturers can reduce production costs and improve overall quality.
Regulatory Compliance and Documentation
Medical device housings must comply with various regulatory standards. Design considerations for compliance include:
- Incorporating features that facilitate easy cleaning and sterilization
- Designing for traceability (e.g., space for serial numbers or QR codes)
- Ensuring designs meet IP (Ingress Protection) ratings if required
- Creating comprehensive technical documentation for regulatory submissions
Maintain detailed design records and documentation to support regulatory approval processes and ensure compliance throughout the product lifecycle.
Quality Control and Validation in CNC Machining Medical Device Housings
Maintaining stringent quality control measures is essential for producing medical device housings that meet industry standards and regulatory requirements. Implement the following best practices to ensure consistent quality:
Implementing Robust Quality Management Systems
Establish a comprehensive quality management system (QMS) that aligns with ISO 13485 standards for medical devices. Key components include:
- Documented procedures for all aspects of the manufacturing process
- Regular internal audits and continuous improvement initiatives
- Training programs for personnel involved in CNC machining operations
- Supplier quality management and material traceability systems
- Risk management processes to identify and mitigate potential issues
A robust QMS ensures consistency in production and helps maintain compliance with regulatory requirements.
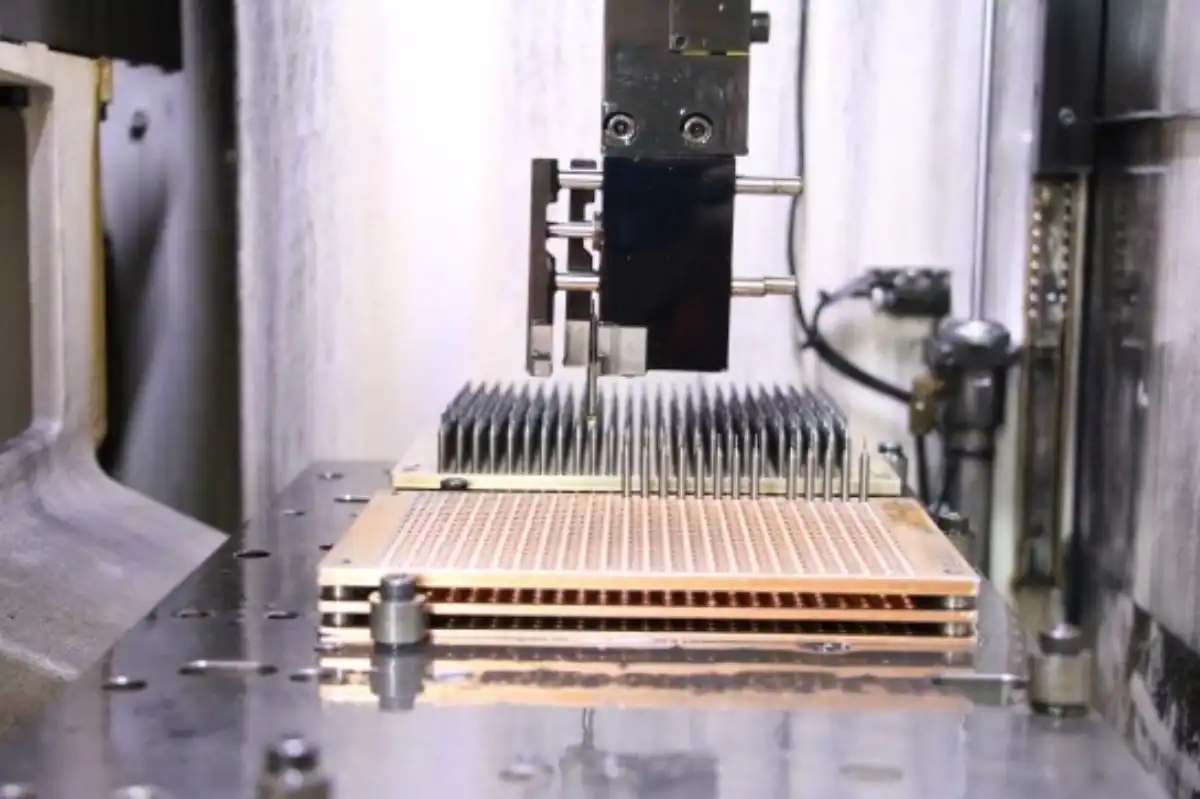
In-Process and Final Inspection Techniques
Implement rigorous inspection protocols throughout the CNC machining process for medical device housings:
- Conduct first article inspections to verify initial production quality
- Perform in-process inspections at critical stages of machining
- Use coordinate measuring machines (CMMs) for precise dimensional verification
- Implement optical inspection systems for surface quality assessment
- Conduct functional testing to ensure proper fit and assembly
Thorough inspection processes help identify and address issues early, reducing the risk of defective products reaching end-users.
Validation and Documentation Practices
Validate CNC machining processes and maintain comprehensive documentation:
- Develop and follow Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols
- Maintain detailed records of machine calibration and maintenance
- Document all process parameters and CNC programs used in production
- Implement a robust change control system for managing design or process modifications
- Create and maintain Device Master Records (DMRs) and Device History Records (DHRs)
Proper validation and documentation practices support regulatory compliance and facilitate continuous improvement efforts in CNC machining medical device housings.
Conclusion
Implementing best practices for CNC machining medical device housings is crucial for producing high-quality, compliant products that meet the stringent demands of the healthcare industry. By focusing on proper material selection, optimized design for manufacturability, and rigorous quality control measures, manufacturers can ensure the production of reliable and safe medical device housings. Adhering to these practices not only enhances product quality but also streamlines regulatory approval processes and builds trust with healthcare providers and patients alike.
FAQs
1. What are the most common materials used for CNC machining medical device housings?
Common materials include medical-grade stainless steel, titanium alloys, PEEK, and medical-grade aluminum alloys.
2. How important is surface finish in medical device housings?
Surface finish is critical as it affects cleanability, biocompatibility, and overall device performance. Smooth surfaces are often required to prevent bacterial growth and facilitate sterilization.
3. What certifications are important for CNC machining medical device housings?
Key certifications include ISO 13485 for medical device manufacturing, FDA compliance, and specific material certifications like USP Class VI for plastics.
Expert CNC Machining for Medical Device Housings | BOEN
At BOEN Prototype, we specialize in high-precision CNC machining for medical device housings. Our state-of-the-art facilities and experienced team ensure top-quality prototypes and low-volume production in various materials. As a trusted supplier and manufacturer, we pride ourselves on meeting the exacting standards of the medical industry. Contact us at contact@boenrapid.com to discuss your medical device housing needs and experience our superior craftsmanship firsthand.
References
Smith, J. (2022). Advanced Materials for Medical Device Housings. Journal of Biomedical Engineering, 45(3), 234-248.
Johnson, A., & Brown, T. (2021). CNC Machining Techniques for Precision Medical Components. Medical Device Manufacturing Technology, 18(2), 112-126.
Garcia, M. et al. (2023). Quality Control Systems in Medical Device Manufacturing. International Journal of Quality Assurance in Healthcare, 32(4), 567-582.
Thompson, R. (2022). Regulatory Compliance in Medical Device Design and Manufacturing. FDA Regulatory Affairs Quarterly, 29(1), 45-60.
Lee, S., & Chen, Y. (2021). Optimization of CNC Machining Parameters for Medical Grade Materials. Journal of Manufacturing Processes, 63, 78-92.
Wilson, K. (2023). Advances in Surface Finishing Techniques for Medical Device Housings. Surface Engineering for Biomedical Applications, 41(5), 301-315.





