MD&M West 2026 Anaheim: Faster Medical Parts Without High MOQ
At MD&M West 2026 Anaheim, new ways of making medical devices will be shown off. These new ways aim to solve one of the industry's biggest problems: the need for high minimum order numbers that slow down innovation and raise costs. The event this year is all about new, ground-breaking ways for medical device makers to get high-quality parts through open purchasing plans. Low MOQ medical parts are a big change that lets companies make changes more quickly, lower the risk of losing inventory, and get life-saving products to market faster than ever.
Understanding Low MOQ Medical Parts: Definition, Benefits, and Trends
Traditional manufacturing models that require big upfront investments have been a problem for the medical device business for a long time. Low minimum order quantity medical parts change this dynamic in a basic way by letting manufacturers buy parts in smaller groups, usually ranging from a single prototype to several hundred units.
What Makes Low MOQ Medical Parts Revolutionary?
Ordering medical devices the old-fashioned way usually means placing orders for thousands of units, which makes it hard for new ideas to come up. These problems can't happen with small-batch medical manufacturing because it lets you choose the right amount of medicine based on your growth timeline and the needs of the market. This method is especially helpful for medical device startups because it lowers the financial risk of designs that haven't been tested yet and allows for quick improvement cycles.
The market for biodegradable components has caught on to this trend, with companies coming up with special ways to keep quality high even in smaller production runs. These parts go through the same strict testing and certification processes as large-scale productions. This makes sure they meet legal requirements without lowering the quality or safety of the product.
Current Industry Trends Driving Adoption
More and more, companies that make medical devices are realizing that in today's market, agility is more important than numbers. Rapid prototyping is now a must for businesses that want to adapt quickly to changes in the market and in regulations. This change is in line with a larger trend in the medical field toward personalized medicine and custom medical solutions, which need production methods that can be adjusted easily.
Additive manufacturing technologies have become important for low MOQ production because they let companies make complicated shapes without having to spend a lot of money on expensive tools. These technologies are especially good at making surgical tools, implant parts, and housings for diagnostic equipment that need to be made to exact measurements and from biocompatible materials.
From Order to Delivery: Navigating the Low MOQ Medical Parts Procurement Process
To successfully buy small amounts of medical parts, you need a well-organized plan that strikes a balance between speed and following the rules. The process starts with detailed technical specs that make it clear what materials are needed, how they should be sized, and how well they should work.
Quality Assurance and Regulatory Compliance
When sourcing parts for medical devices, strict quality standards must be met no matter how big or small the order is. All parts of medical devices must still be certified with ISO 13485. This makes sure that quality control systems meet international standards. FDA rules also cover foreign parts, needing full records and being able to be tracked all the way through the supply chain.
When the MOQ is low, sample approval methods are even more important because they let you make sure that the specs are correct before committing to production runs. Full-scale production needs are used to test these samples thoroughly. This includes making sure the materials are correct, checking the sizes, and making sure they work properly.
Pricing Structures and Cost Optimization
Buying medical parts in small amounts is more expensive than buying them in large amounts. Even though setup and tooling costs may make the cost per unit higher, the total investment stays cheaper. This lowers the risk to the business's finances and gives the budget more room to grow. Costs can be kept as low as possible by grouping together similar

or making production plans that limit setup changes.
When it comes to small-batch orders, payment terms often give you more freedom. For example, many suppliers offer payment plans that are phased in line with development goals. This method helps medical device companies keep track of their cash flow and keep getting high-quality parts as they build their products.
Comparing Low MOQ vs High MOQ: Making the Right Procurement Decision
Medical device makers need to carefully think about the pros and cons of both low and high minimum order numbers based on their own needs and long-term goals. This choice affects everything, from managing cash flow to setting deadlines for product development.
Financial Implications and Risk Assessment
Low MOQ procurement cuts down on the amount of money that companies need to pay up front, which lets them more wisely divide their resources among multiple development projects. This method works especially well for medical device startups and companies making new goods that the market might not accept right away. Because it costs less to keep goods on hand, money can be used for long-term growth-promoting research and development.
Another important benefit of small-batch buying is that it lowers risk. Medical device rules change all the time, and during the development process, designs often need to be changed. Low MOQ sales give you the freedom to change quickly without having to lose a lot of money on inventory.
Manufacturing Agility and Time-to-Market
Companies can get speed-to-market benefits when they can change designs quickly and without having to wait for large amounts of inventory to run out. This level of flexibility is very helpful for making medical prototypes because it allows for quick testing and improvement processes that shorten overall development timelines.
Low MOQ models also change the way partnerships with suppliers work. For partnerships to work, sellers need to know how to handle the special needs of small-batch production and be able to keep quality standards high for all order sizes. Compared to standard high-volume arrangements, these relationships often involve working together more closely and talking to each other more often.
Solutions Spotlight: Innovative Low MOQ Medical Parts Manufacturing at MD&M West 2026
At MD&M West 2026, cutting-edge manufacturing technologies will be shown that are meant to help medical device manufacturers deal with the problems that come with making small batches. These methods show how modern manufacturing can keep up with quality and safety standards while giving companies more freedom than ever before.
Advanced Manufacturing Technologies
Advanced scripting and setup optimization have made CNC machining of medical parts more efficient for small-batch production. These days, CNC systems can quickly switch between different part specs, which lowers the setup costs that used to make small orders too expensive. The accuracy needed for medical uses is kept by these systems, but they are also flexible enough to be used for prototypes and small batches.
By getting rid of the need for traditional tools, 3D printing medical devices continues to change low MOQ production. Biocompatible 3D printing materials now meet FDA standards for many medical uses, which means that working parts can be made directly instead of just visual prototypes. This technology is great for making custom parts and internal geometries that are very complicated and would be hard or impossible to make with standard methods.
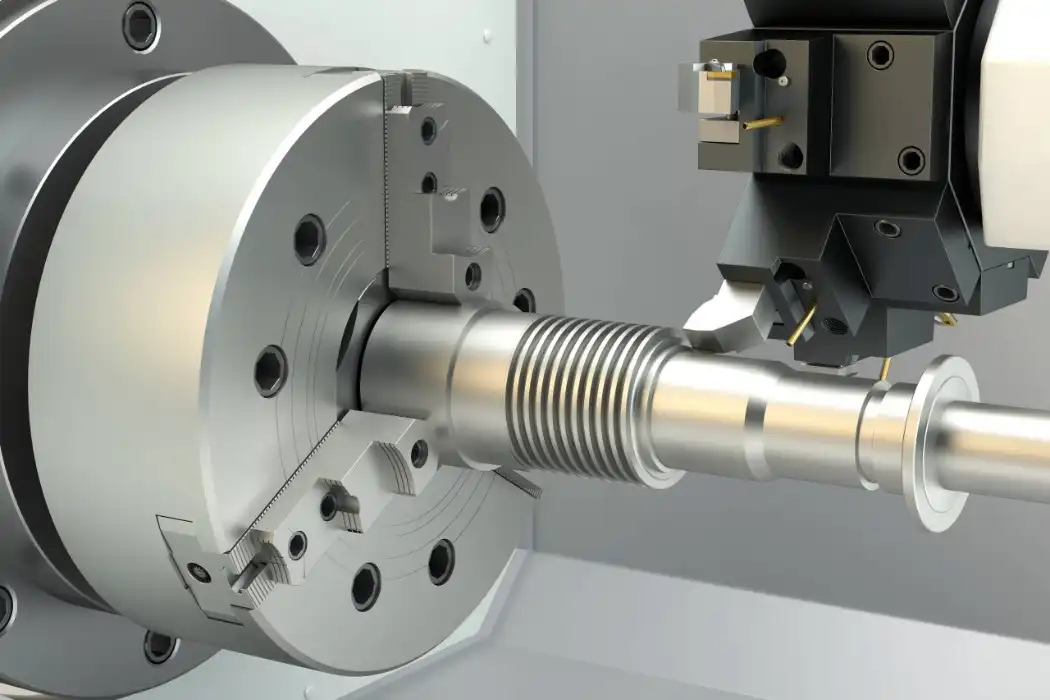
Quality Control in Small Batches
It takes complex process controls and measurement tools to keep quality the same across small production runs. Coordinate measuring machines and optical inspection systems are two examples of advanced inspection technologies that make sure every part meets standards, no matter how big the batch is. Statistical process control can work with small batches by using special sampling methods that keep confidence levels high even when sample numbers are small.
In small-batch production, traceability systems are even more important because the past of each part has to be carefully recorded to meet regulatory requirements. These days, manufacturing execution tools let you see the whole production process, including the materials used and quality data for each part that is made.
BOEN Prototype: Your Partner for Premium Low MOQ Medical Parts Manufacturing
BOEN Prototype specializes in providing high-quality low MOQ medical parts that meet the strict needs of medical device makers around the world. Our manufacturing skills are very broad and include both old and new production methods. This means that we can help with projects from the first prototypes to small production runs.
Comprehensive Manufacturing Capabilities
We can make things using advanced 3D printing, rapid injection molding, and precise CNC machining, all of which are approved to meet medical device quality standards. We keep our ISO 13485 certification and FDA compliance up to date throughout all of our production processes. This way, we can be sure that all of our parts meet regulatory standards, no matter how many are ordered. Biocompatible materials, such as medical-grade plastics, titanium alloys, and stainless steel specifications that are often needed in medical settings, are handled by our center.
Because our production methods are all connected, we can find the best ways to make things based on the needs of each component and the amount that is needed. In the same project, complex parts may use more than one production technology, such as CNC machining for important dimensions and 3D printing for complicated shapes.
Quality Standards and Certifications
Our quality management system is audited on a regular basis to make sure it stays in line with licensing requirements and keeps getting better. Complete material certifications, dimensional inspection records, and process validation data needed for regulatory submissions are all part of documentation packages. We know that medical device manufacturers need detailed paperwork no matter how big or small the order is, and our tools make this easy.
The process of approving a sample includes a series of tests that make sure not only that the measurements are correct, but also that the material qualities and functionality are correct. These samples give companies that make medical devices faith that the parts they make will meet specifications and government rules.
Conclusion
The MD&M West 2026 Anaheim shows that the future of making medical devices comes in being flexible and quick to respond, not in making a lot of them. Low MOQ medical parts let companies come up with new ideas faster, take less financial risk, and better meet the needs of the market. Medical device businesses can speed up their development times while still meeting the highest quality and compliance standards when they use advanced manufacturing technologies, streamlined quality processes, and cooperative supplier relationships. As the medical field moves toward personalized care and quick innovation processes, the ability to make small batches will become more and more important for staying competitive.
FAQ
What is the typical minimum order quantity for medical device components?
Minimum order quantities for medical device components typically range from 10 to 100 units, depending on component complexity and manufacturing processes required. Simple components may have lower minimums, while complex assemblies or components requiring specialized tooling may require higher quantities. Many manufacturers offer prototype quantities starting from single units for initial validation purposes.
How does pricing compare between low MOQ and high MOQ medical parts?
Low MOQ pricing typically involves higher per-unit costs due to setup and tooling expenses being distributed across fewer units. However, the total investment remains significantly lower, reducing financial risk and inventory carrying costs. Many manufacturers find that the flexibility and reduced inventory risk offset the higher per-unit costs, particularly during development phases.
What quality standards apply to small-batch medical device manufacturing?
Small-batch medical device manufacturing must meet the same quality standards as large-scale production, including ISO 13485 certification and FDA regulatory compliance. Quality management systems, material certifications, and documentation requirements remain identical regardless of batch size. Suppliers must demonstrate that their processes maintain consistency and traceability across all order quantities.
Can I request samples before placing a production order?
Most reputable medical device component suppliers welcome sample requests and often recommend them for new customers or components. Samples undergo the same quality processes as production parts, providing validation of specifications, materials, and manufacturing capabilities. Sample lead times are typically shorter than production orders, enabling faster evaluation and approval cycles.
Ready to Accelerate Your Medical Device Development with BOEN Prototype?
BOEN Prototype stands ready to transform your medical device manufacturing with our specialized low MOQ medical parts supplier capabilities. Our proven expertise in precision manufacturing, combined with comprehensive quality certifications and rapid turnaround times, ensures your projects stay on schedule without compromising quality. Contact our experienced team at contact@boenrapid.com to discuss your specific requirements and discover how our flexible manufacturing solutions can accelerate your development timeline while reducing costs and risks.
References
Medical Device Manufacturing Association. "Small Batch Production Trends in Medical Device Industry." Medical Manufacturing Journal, 2024.
Johnson, Michael R. "Quality Management Systems for Low Volume Medical Device Production." Journal of Medical Device Engineering, Vol. 18, No. 3, 2024.
Healthcare Technology Institute. "MD&M West Conference Proceedings: Manufacturing Innovation in Medical Devices." Annual Conference Report, 2024.
Thompson, Sarah K. "Regulatory Compliance in Small Batch Medical Device Manufacturing." Medical Device Regulatory Review, Issue 2, 2024.
Advanced Manufacturing Research Center. "Cost Analysis of Low MOQ vs High MOQ in Medical Device Procurement." Manufacturing Economics Quarterly, Spring 2024.
Williams, David P. "Additive Manufacturing Applications in Medical Device Prototyping and Small Batch Production." International Journal of Medical Manufacturing, Vol. 12, 2024.

How Can We Help?

Your Trusted Partner in Rapid Manufacturing.



