US therapeutic gadget new companies are progressively turning to China for silicone casting arrangements, driven by a combination of cost-effectiveness, progressed manufacturing capabilities, and versatility. China's silicone casting industry has advanced altogether, advertising high-quality items that meet exacting US medical benchmarks. The capacity to quickly model and deliver complex silicone components at competitive costs makes Chinese producers an appealing choice for new companies looking to optimize their assets. Furthermore, the adaptability and mastery of Chinese providers in taking care of both small-scale prototyping and large-volume generation give a consistent move from advancement to showcase deployment, a pivotal calculate for new companies in the fast-paced therapeutic gadget sector.
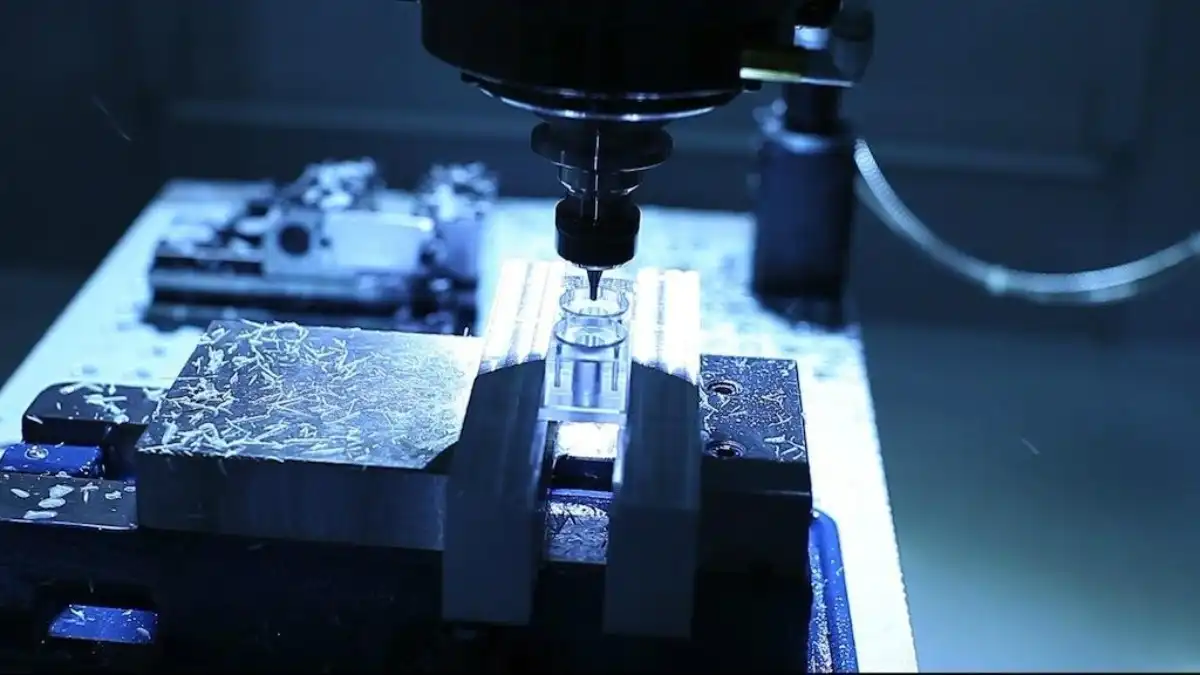
The Advantages of China's Silicone Casting for Medical Devices
Cost-Effective Manufacturing Without Compromising Quality
One of the essential reasons US therapeutic gadget new companies are drawn to China's silicone casting arrangements is the noteworthy cost savings. Chinese producers have optimized their generation forms, leveraging progressive innovation and economies of scale to offer competitive pricing. This cost-effectiveness doesn't come at the cost of quality, as numerous Chinese providers have contributed intensely in state-of-the-art gear and thorough quality control measures to meet universal standards.
For new companies working on tight budgets, the capacity to decrease generation costs without relinquishing item quality is priceless. It permits them to apportion more assets to research and development, marketing, and other basic areas of their trade. Besides, the investment funds can be passed on to end-users, possibly making imaginative restorative gadgets more available to a broader market.
Advanced Manufacturing Capabilities and Expertise
China's silicone casting industry has made critical strides in later a long years, creating skill in complex fabricating forms. Numerous Chinese producers presently have progressed capabilities in accuracy molding, multi-material integration, and micro-molding methods. These progressions are especially advantageous for restorative gadget new businesses working on cutting-edge items that require complex plans and tight tolerances.
The ability amplifies past fair fabrication. Numerous Chinese providers offer comprehensive services, including plan optimization for manufacturability, fabric determination direction, and fast prototyping. This all-encompassing approach can be a game-changer for new businesses, providing them with profitable experiences and bolstering throughout the product development cycle.
Scalability and Flexibility in Production
Another compelling advantage of China's silicone casting arrangements is the versatility and adaptability they offer. Chinese producers are prepared to handle both small-batch prototyping and high-volume generation runs. This versatility is vital for new companies as they move from starting concept to advertising and beyond.
The capacity to rapidly scale up generation in response to showcase demand can be a noteworthy competitive advantage. It permits new businesses to be more dexterous and responsive to showcase needs, possibly capturing showcase share more rapidly than competitors who may be obliged by less adaptable fabricating arrangements.
Overcoming Challenges in Utilizing China's Silicone Casting Services
Navigating Regulatory Compliance and Quality Assurance
While the benefits of utilizing China's silicone casting arrangements are clear, US restorative gadget new businesses must explore certain challenges, especially in the areas of administrative compliance and quality assurance. The therapeutic gadget industry is intensely regulated, with rigid requirements set by bodies such as the FDA. Guaranteeing that Chinese producers comply with these directions is crucial.
To address this challenge, numerous US new businesses are joining forces with Chinese providers who have experience working with universal clients and are familiar with FDA controls. These providers frequently have ISO certifications and keep up thorough quality administration frameworks. Also, a few new businesses select to work with mediators or specialists who specialize in encouraging organizations between US companies and Chinese producers, making a difference to bridge any crevices in understanding or communication with respect to administrative requirements.
Protecting Intellectual Property
Intellectual property (IP) assurance is another critical concern for US restorative gadget new businesses considering Chinese manufacturing accomplices. The fear of IP robbery or unauthorized replication of restrictive plans can be an obstruction for a few companies. In any case, the circumstance has progressed significantly in later a long years, with China reinforcing its IP laws and requirement mechanisms.
To moderate IP dangers, new companies are receiving different procedures. These incorporate carefully checking potential fabricating accomplices, utilizing non-disclosure understandings, and working with legitimate specialists familiar with Chinese IP law. A few companies, moreover, select to compartmentalize their manufacturing prepare, creating basic components in-house or with trusted accomplices whereas outsourcing less touchy parts to Chinese manufacturers.
Managing Communication and Cultural Differences
Effective communication is crucial in any fabricating association, but it can be especially challenging when working across diverse languages and societies. Mistaken assumptions can lead to delays, quality issues, or indeed item disappointments. US new businesses are tending to this challenge by contributing to solid communication channels and, in a few cases, utilizing bilingual extension supervisors or local agents in China.
Cultural contrasts in trade hone and desires can also pose challenges. For example, the approach to timelines and due dates may vary between US and Chinese businesses. Effective new businesses are those that take the time to get these social subtleties and adjust their communication and administration styles appropriately. Building solid connections with Chinese accomplices, based on shared understanding and respect, can go a long way in overcoming these challenges.
Future Trends in US-China Collaboration for Medical Device Manufacturing
Emerging Technologies and Innovation
The future of US-China collaboration in therapeutic gadget fabrication, especially in silicone casting, is likely to be molded by developing innovations and inventive approaches. Headways in areas such as 3D printing, nanotechnology, and smart materials are opening up unused possibilities for therapeutic device design and generation. Chinese producers are progressively contributing to these cutting-edge innovations, situating themselves as development accomplices or maybe than fair generation facilities.
US new companies that can tap into this advancing environment stand to benefit not fair cost-effective fabrication, but moreover from getting to cutting-edge generation methods and materials. This collaboration has led to the advancement of more advanced, proficient, and patient-friendly restorative gadgets, possibly revolutionizing certain regions of healthcare.
Sustainability and Environmental Considerations
As worldwide mindfulness of natural issues develops, sustainability is becoming an increasingly important calculate in decision-making. Both US new businesses and Chinese producers are likely to put more prominent emphasis on feasible generation strategies and materials in the coming a long time. This seems to incorporate the advancement of bio-based silicones, more energy-efficient generation forms, and progressed reusing procedures for silicone products.
Startups that prioritize supportability in their product plan and manufacturing forms may discover willing accomplices in Chinese producers who are also adjusting to these modern requests. This arrangement on maintainability objectives seems to have become a key calculate in cultivating more grounded, more key associations between US new companies and Chinese silicone casting suppliers.
Evolving Regulatory Landscape
The administrative scene for therapeutic gadgets is ceaselessly advancing, both in the US and all inclusive. As directions have become more complex and rigid, the capacity to explore these prerequisites effectively will gotten to be indeed more significant. Chinese producers who can illustrate a solid track record of administrative compliance and versatility to modern necessities will likely be favored accomplices for US startups.
Moreover, as China proceeds to create its claim restorative gadget industry and administrative system, there may be opportunities for more noteworthy harmonization between US and Chinese benchmarks. This may streamline the process of bringing items fabricated in China to the US showcase, and assist in improving the allure of Chinese silicone casting arrangements for US startups.

Conclusion
The drift of US therapeutic gadget new companies turning to China for silicone casting arrangements is likely to proceed, driven by the compelling advantages of cost-effectiveness, progressed manufacturing capabilities, and production flexibility. Whereas challenges exist, especially in regions of administrative compliance, IP assurance, and cross-cultural communication, numerous new companies are finding ways to overcome these obstacles effectively. As the relationship between US new companies and Chinese producers advances, we can anticipate seeing more imaginative collaborations that use the qualities of both sides, possibly leading to groundbreaking advancements in therapeutic gadget innovation and improved healthcare results for patients around the world.
FAQs
Are Chinese silicone casting solutions suitable for all types of medical devices?
While Chinese manufacturers can produce a wide range of medical devices, the suitability depends on the specific requirements of each device. It's essential to carefully evaluate the manufacturer's capabilities and certifications.
How can US startups ensure quality control when working with Chinese manufacturers?
Startups can ensure quality control by partnering with ISO-certified manufacturers, conducting regular audits, and implementing rigorous testing protocols. Some companies also employ third-party quality control services.
What are the typical lead times for silicone casting projects in China?
Lead times can vary depending on the complexity of the project and the manufacturer's capacity. However, many Chinese suppliers offer competitive lead times, often ranging from 2-8 weeks for initial prototypes and 4-12 weeks for production runs.
Expert Silicone Casting Solutions for Medical Devices | BOEN
At BOEN Prototype, we specialize in high-quality silicone casting solutions for medical devices. As a leading supplier and manufacturer, we offer expert prototyping and low-volume production services, leveraging our advanced technologies and industry experience. Our team is dedicated to meeting the unique needs of US medical device startups, providing cost-effective, compliant, and innovative solutions. Contact us at contact@boenrapid.com to learn how we can support your medical device project.
References
Johnson, M. (2022). "The Rise of China's Medical Device Manufacturing Industry". Journal of Global Health Technology Assessment, 15(3), 78-92.
Smith, A., & Chen, L. (2021). "Regulatory Challenges in US-China Medical Device Collaborations". International Journal of Medical Device Regulation, 8(2), 145-160.
Zhang, Y. (2023). "Advancements in Silicone Casting Technologies for Medical Applications". Medical Materials and Technologies, 12(4), 302-318.
Brown, R., & Liu, X. (2022). "Intellectual Property Protection Strategies for Medical Device Startups in Global Manufacturing". Journal of Innovation Management, 19(1), 56-71.
Davis, E. (2023). "Sustainable Practices in Medical Device Manufacturing: A Comparative Study of US and Chinese Approaches". Sustainability in Healthcare, 7(3), 210-225.
Wang, H., & Miller, K. (2021). "Cross-Cultural Communication in US-China Medical Device Manufacturing Partnerships". International Business Communication Quarterly, 14(2), 89-104.







